Lab 4 – Background Reading
Photosynthesis
Organisms that can create their own food are known as autotrophs. While plants have this ability, humans do not, and as a result all of our energy initially comes from plants, and ultimately, from the sun. Plants make their own food using light energy from the sun. Specifically, this process is known as photosynthesis, and plants use this process to make a type of carbohydrate called glucose. Carbohydrates are a type of macromolecule (nucleic acids, proteins, and lipids are the other three) made up of only carbon, hydrogen, and oxygen, and these are always found in a 1:2:1 ratio. For example, the chemical formula for glucose is C6H12O6, where you can see that the ratio of carbon : hydrogen : oxygen is 1:2:1. In other words, the number of hydrogens in a carbohydrate is always double the number of carbons and oxygens. Through photosynthesis, plants uptake carbon dioxide (CO2), water (H2O), and sunlight energy, and convert them into oxygen (O2) and glucose (C6H12O6). The chemical equation for photosynthesis is:
6 CO2 + 6 H2O + sunlight –> C6H12O6 + 6 O2
Notice the sixes? Those are to balance the equation. For every one molecule of glucose a plant produced through photosynthesis, it needed six molecules of CO2 and six molecules of H2O (along with sun energy) to make that happen. To get the reactants needed for photosynthesis, plants uptake water from their roots, and acquire carbon dioxide from the air via openings in the leaves called the stomata (sometimes called the stoma, but not to be confused with stroma). The sunlight comes from, quite obviously, the sun.
As you will discuss in class, the organelle in plant cells responsible for photosynthesis is the chloroplast. Inside the chloroplast, which has an inner and outer membrane, the highly folded thylakoid (from the Greek thylakois for “sac”) membranes form structures that resemble stacks of sacs. Thylakoids are stacked in groups called grana (singular: granum), which look like stacks of interlinked pancakes. The space inside the chloroplast that surrounds the grana is a gel-like fluid known as the stroma. Photosynthesis occurs through a series of chemical reactions known as the light reactions, which occur in the thylakoid, and the Calvin Cycle (sometimes referred to as the “dark reactions”), which occurs in the stroma.
Sunlight and water combine in the light reactions to produce oxygen gas and two molecules: ATP and NADPH. Adenosine triphosphate, known as ATP, is a large molecule that is a form of energy used by cells, specifically, cells use the energy stored in the chemical bonds of ATP. Nicotinamide adenine dinucleotide phosphate (NADPH), along with its oxidized form NADP+ (a molecule that is oxidized has lost electrons, so NADP+ is the oxidized form of NADPH because it is missing the electrons from the Hydrogen) are molecular energy carriers that transfer electrons (i.e. energy) between the two photosynthetic processes. Carbon dioxide, along with ATP and NADPH, power the Calvin Cycle, which produces glucose, and two other molecules: ADP, or adenosine diphosphate, and NADP+. Adenosine diphosphate is the result of breaking one of the bonds in ATP to remove one phosphate, reducing the number of phosphates from three to two. Remember, that energy is stored in chemical bonds, so some of the energy that powers the Calvin Cycle comes from breaking one bond in ATP. Energy inputs during the light reactions reform ADP and NADP+ as ATP and NADPH, and again ATP and NADPH’s chemical bonds are broken during the Calvin Cycle and used as energy to power cellular activities. Think of ATP and NADPH as rechargeable batteries that release energy when one of their chemical bonds is broken, and hold energy when that bond is reformed.
Notice that the Calvin Cycle does not use light directly, hence the name “dark reactions”; you might have even heard it referred to as the “light independent reactions”. However, the Calvin Cycle is powered by a steady supply of NADPH and ATP (along with inputs of CO2 that come from outside the plant) that are supplied by the light reactions, so when a plant’s light source is removed, the Calvin Cycle will use up what is left of the available NADPH and ATP, after which it will stop until the light source is present once again.
Photosynthesis and the Electromagnetic Spectrum
Figure 2. Colors and their corresponding wavelengths of light. Source: Humboldt University Geospatial Analysis Brochure, 2014.
The energy that powers photosynthesis is ultimately provided by the sun, in the form of photons, which have wavelike characteristics. The energy contained in a photon corresponds to the size of its wavelength, which is the distance between one wave crest (the top of the wave) and the next. Photons come in a wide range of wavelengths, known as the electromagnetic spectrum (Figure 1), and shorter wavelengths contain more energy than longer wavelengths. The part of the electromagnetic spectrum that is visible to us is the visible light spectrum, and it includes wavelengths from 300 nanometers (nm) to 780 nm. Visible light spans the rainbow from violets (300 nm) to reds (780 nm) (Figure 2).
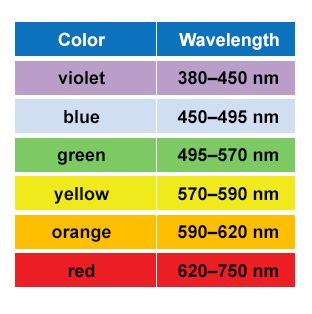
The color of an object is determined by the wavelengths of light that is absorbs and of those that bounce off of it. Objects that appear red, like a stop sign, absorb the wavelengths of all the light colors except red, meaning that a red stop sign reflects wavelengths in the range of 620 – 750 nm and absorbs all others in the visible light spectrum. White objects reflects all wavelengths (hence why it is known as “white light”) in the visible light spectrum (white light = 380 – 700 nm), while black objects absorb all of these wavelengths. The absorption of wavelengths of light is why wearing a black shirt on a sunny day feels much hotter than wearing a white shirt. The black shirt absorbs all the wavelengths of light, while the white shirt reflects them.
Chlorophyll is the main light absorbing pigment found in the thylakoids of plant cells, and the wavelengths of light absorbed by this green pigment drive the light reactions of photosynthesis. While they tend to be grouped together, chlorophyll is really two separate pigments of chlorophyll a and chlorophyll b, and each of these absorbs lights at slightly different wavelengths. Collectively they harvest light over a broader range than just one pigment could (Figure 3).
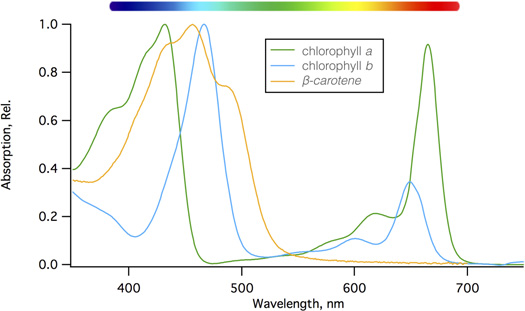
Other pigments such as yellow-orange carotenoids (kuh-RAH-tuh-noyds) and colorful flavonoids also exist in plant cells. Carotenoids help harvest light in plant cells, and have the additional function of protecting photosynthetic cells from too much sun; they act as a safety valve for excess absorbed light energy, which they release as heat. In cold parts of the world, broad-leaved plants drop their leaves in autumn to protect against excessive water loss during the cold seasons. The breakdown of chlorophyll in the leaves reveals the carotenoids, which linger to protect the cells as the programmed dismantling of the foliage continues. Carotenoids are responsible for the beautiful orange and yellow leaves, while flavonoids are responsible for red leaves that are classically associated with autumn.
Cellular Respiration
Aerobic (oxygen dependent) cellular respiration is the process by which glucose and oxygen are converted to carbon dioxide, water, and energy in the form of ATP by the mitochondria in eukaryotic cells. Cellular respiration is a three-step process of (1) glycolysis, (2) the Krebs Cycle, and (3) the electron transport chain (ETC). The first step, glycolysis, occurs within the cytoplasm outside the mitochondria, and through this process, which also requires oxygen, glucose is split to create pyruvate, a three-carbon compound. Glycolysis releases two molecules of ATP and two molecules of NADH (an energy transporter) for each molecule of glucose that is split, as well as some CO2. In the second step, pyruvate enters the mitochondrion and begins the Citric Acid Cycle (sometimes called the Krebs Cycle). Here, the energy released from breaking the bonds of pyruvate are used to produce multiple energy carriers, such as ATP and NADPH, as well as more CO2. In the final step, the Electron Transport Chain (ETC; aka oxidative phosphorylation), the NADPH from the first two steps releases its energy, and through a chemical reaction these are converted to ATP. This step also releases water as a byproduct. The final step is the most productive, with the ETC generating about 34 ATP. At maximum, each molecule of glucose used up in cellular respiration can create 38 ATP.
C6H12O6 + 6 O2 –> 6 CO2 + 6 H2O + energy
You might have realized that the equation for cellular respiration (above) looks really familiar – and it should. If we replace “energy” with “sunlight” in the photosynthesis equation, it should be evident that cellular respiration and photosynthesis are inversely related. Photosynthesis takes energy and converts it to glucose, while cellular respiration takes glucose and converts it to energy. Remember that plant cells are eukaryotic cells, so they have mitochondria. This makes plant cells capable of performing both photosynthesis and cellular respiration. When light is available, the rate of photosynthesis exceeds the rate of cellular respiration, however, when no light is available and the light reactions of photosynthesis cease, the mitochondria of plants continue to create energy through cellular respiration – which means at night your houseplants compete with you for oxygen!
References
- Singh-Cundy, A. and G. Shin. 2015. Discover Biology, 6th ed. W. W. Norton & Company Inc. New York, New York.
